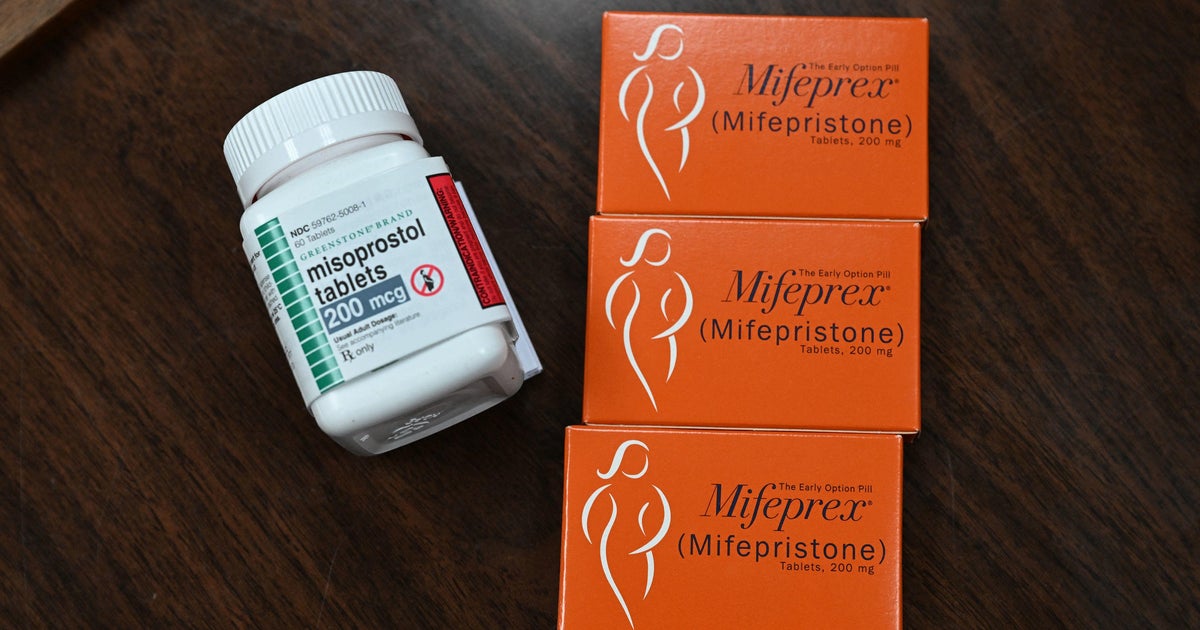
Washington — A federal judge on Friday halted the Food and Drug Administration’s approval of the abortion pill mifepristone, delivering a blow to abortion rights advocates in the wake of the Supreme Court’s dismantling of the constitutional right to abortion.
In a 67-page opinion, U.S. District Judge Matthew Kacsmaryk said the FDA’s two-decades old approval violated federal rules that allow for accelerated approval for certain drugs and subsequent actions by the agency ran afoul of federal law. He put his decision on hold for seven days to allow for the Biden administration to appeal to the U.S. Court of Appeals for the 5th Circuit.
The decision from Kacsmaryk, appointed by former President Donald Trump, further restricts abortion nationwide amid a legal landscape that has been upended since the Supreme Court reversed Roe v. Wade last June. At least a dozen states have enacted near-total bans on the procedure or imposed tighter restrictions in the wake of the decision to unwind the right to an abortion under the U.S. Constitution.
The FDA approved mifepristone more than 20 years ago, and the drug is taken together with a second medicine, misoprostol, to terminate a pregnancy through 10 weeks gestation. Since then, the agency has made several changes to the rules surrounding the abortion pill, including approving a generic version of mifepristone in 2019 and lifting a requirement that the pills be dispensed in-person in 2021, which allowed the drug to be prescribed by a provider during telemedicine appointments and sent by mail.
But it wasn’t until last November, 22 years after the FDA’s approval of the abortion pills, that a group of physicians and medical associations filed a lawsuit seeking to undo the agency’s approval of mifepristone. The lawsuit was filed in the federal district court in Amarillo, where only one judge, Kacsmaryk, is assigned cases.
In their complaint filed by the Alliance Defending Freedom, a conservative legal organization, the doctors argued the FDA erred in determining the abortion drug’s safety and effectiveness and approving it under a federal rule that allows accelerated approval of certain drugs that treat “serious or life-threatening illnesses.” The challengers claim the agency exceeded its regulatory authority to approve the mifepristone and asked the court to issue a preliminary injunction ordering the FDA to undo its approval of mifepristone.
Kacsmaryk held a hearing to consider their request last month.
“The [FDA] must protect the health, safety, and welfare of all Americans by rejecting or limiting the use of dangerous drugs. But the FDA failed America’s women and girls when it approved chemical abortion drugs for use in the United States,” the anti-abortion rights physicians and medical associations told the court. “And it has repeatedly failed them by removing even the most basic precautionary requirements associated with their use.”
But in urging the court to keep the approval of the drug in place, the FDA argued the plaintiffs waited too long to fight its approval of the abortion drug, as challenges to agency actions are subject to a six-year statute of limitations. The agency also noted that while the lawsuit claims the FDA’s approval of mifepristone involved an accelerated review, the drug received the FDA’s OK more than four years after its application from manufacturer Danco Laboratories was submitted.
“Removing access to mifepristone would cause worse health outcomes for patients who rely on the availability of mifepristone to safely and effectively terminate their pregnancies,” the FDA told the court, adding that the “sudden absence” of medication abortion would likely impose “real and significant harms” to patients choosing to take abortion pills out of medical necessity, for privacy or to avoid further trauma.
The FDA also warned that removing the option of medication abortion would lead to overcrowding and delays at clinics already grappling with more patients navigating abortion restrictions in neighboring states.
“This would lead to delays for an array of healthcare services as providers and resources are unnecessarily diverted to surgical abortions,” the agency said.
The challenge to the abortion drug is the latest effort from anti-abortion rights advocates to limit access. In addition to abortion bans by gestational age and method, Republican-led states have also enacted laws limiting medication abortion.
Near-total abortion bans in 12 states supersede restrictions on medication abortion, while 15 states require medication abortion be provided by a physician. In six states, the patient must have an in-person visit with a medical practitioner, according to the Guttmacher Institute, a research group that supports abortion rights.
At the federal level, the Biden administration has taken steps to expand the availability of abortion pills. The FDA in January finalized a rule change that broadens availability of the drugs by allowing more retail pharmacies to dispense the drugs to patients with a prescription. Walgreens and CVS then said they intend to sell mifepristone.
The Justice Department’s Office of Legal Counsel also gave the green-light for the U.S. Postal Service to mail mifepristone and misoprostol, finding that the Comstock Act of 1873 does not prohibit the mailing of the drugs.
Medication abortions have become more common over the years, accounting for more than half of all abortions in the U.S. in 2020, according to the Centers for Disease Control and Prevention.
The case targeting mifepristone attracted significant public interest and scrutiny of Kacsmaryk, largely focusing on his conservative views and the motivation behind the suit being filed in Amarillo.
Critics of the anti-abortion rights groups have accused them of engaging in forum-shopping, a practice in which a party will pursue a claim in the court most likely to be favorable to them. Kacsmaryk also came under criticism after he delayed notifying the public of a hearing on March 15.
(Except for the headline, this story has not been edited by PostX News and is published from a syndicated feed.)